
Liquid biopsies provide better therapy options for more patients
Hedera Dx is dedicated to transforming oncology by providing cutting-edge liquid biopsy solutions that improve patient experiences, outcomes and journeys, as well as healthcare professional experiences, while facilitating the prescription, discovery and development of the next breakthrough precision oncology therapies.
Hedera Profiling 2 ctDNA test panel
A compendium of pure clinical actionability
Hedera Profiling 2 ctDNA test panel is a compact ctDNA based liquid biopsy assay for profiling of most common solid tumors.
Compact but Comprehensive
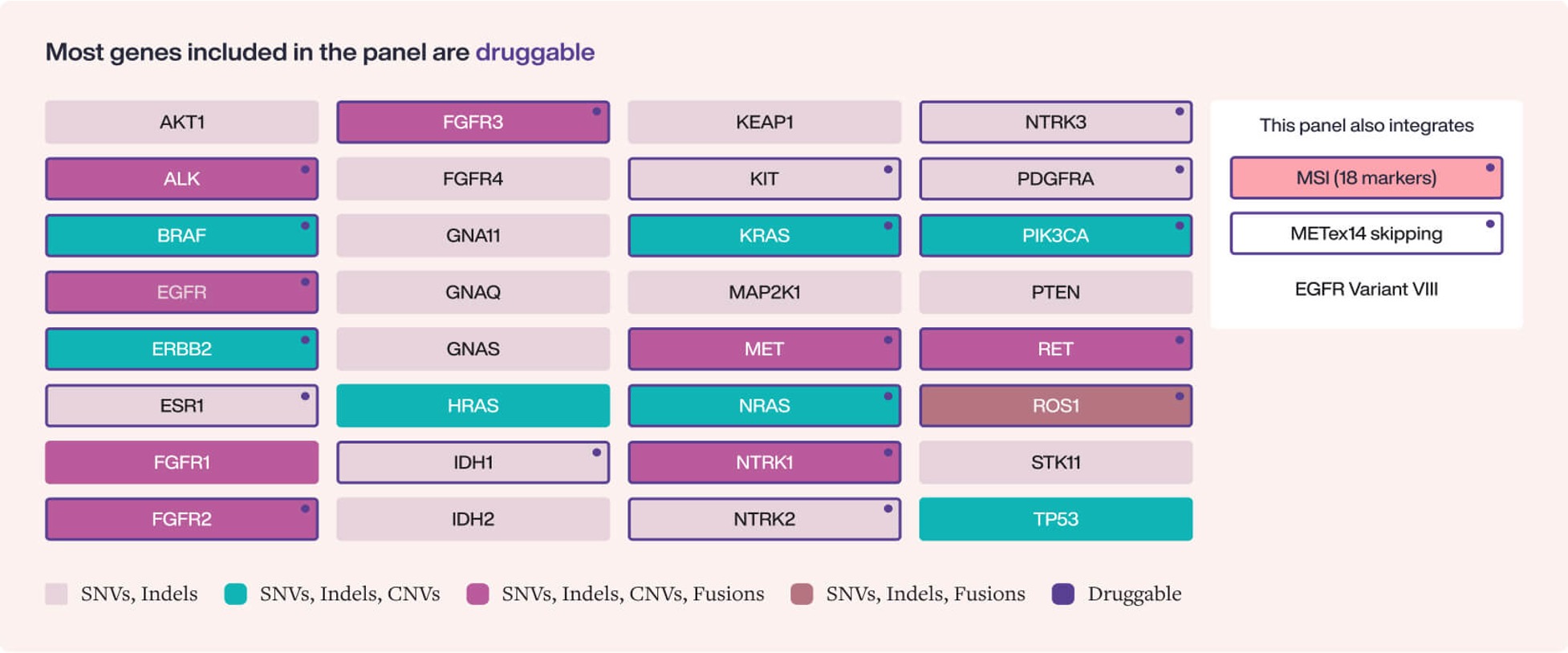
32 gene-panel for the detection SNVs, Indels, CNVs, Fusions and MSI across > 6 most common tumor types in a single, DNA-only liquid biopsy assay.
Robust Software Analysis
Backed by Hedera Prime, a registered IVD Medical Device Software, save time on each report while ensuring accurate and reliable results.
IVDR Compliance
Tailored expert IVDR implementation program for local compliance, facilitating routine testing with in-house validation support meeting local requirements.
Hedera Profiling 2 ctDNA test panel : a compendium of pure clinical actionability
The assay’s unique design allows it to assess a broad range of biomarkers, including SNVs, Indels, CNVs, Fusions and MSI in a single DNA-only, streamlined and robust lab-to-report workflow. It contains >80% of all ESCAT Level I genes included to date in the guidelines.
> 80% of all ESCAT Level I genes are included in the Hedera Profiling 2 ctDNA test panel*
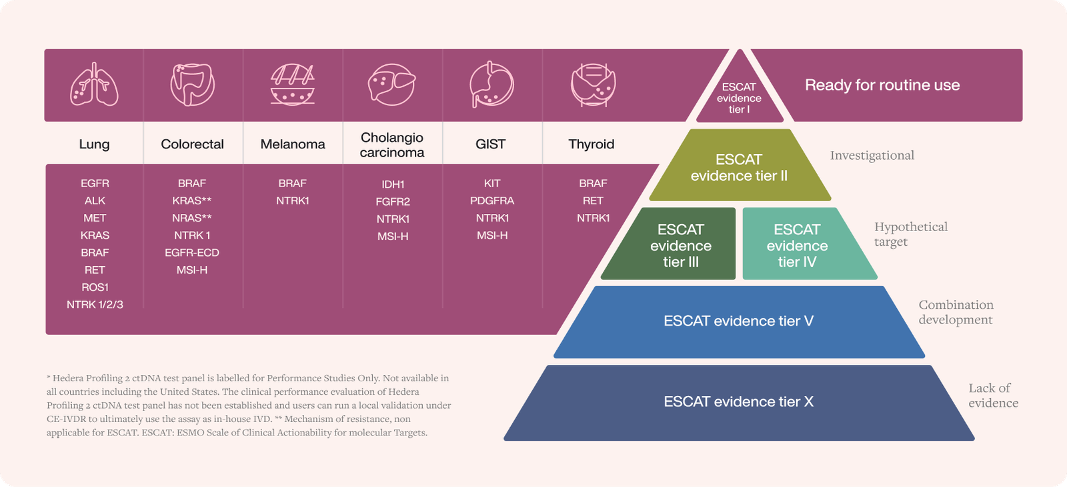
Hedera Profiling 2 ctDNA test panel includes SNVs, Indels, CNVs, Fusions, and MSI
The panel offers highly sensitive and specific variant detection with 30 ng total cfDNA input, capable of identifying variants of high clinical interest, including those classified ESCAT Level I, at 0.5% VAF.
Results can be obtained within a swift 5 calendar days enabling labs to expedite testing timelines without compromising data accuracy, achieving an overall panel sensitivity > 97% and specificity > 99% at 0.5% VAF for key variants classified ESCAT Level I, across multiple cancer types.
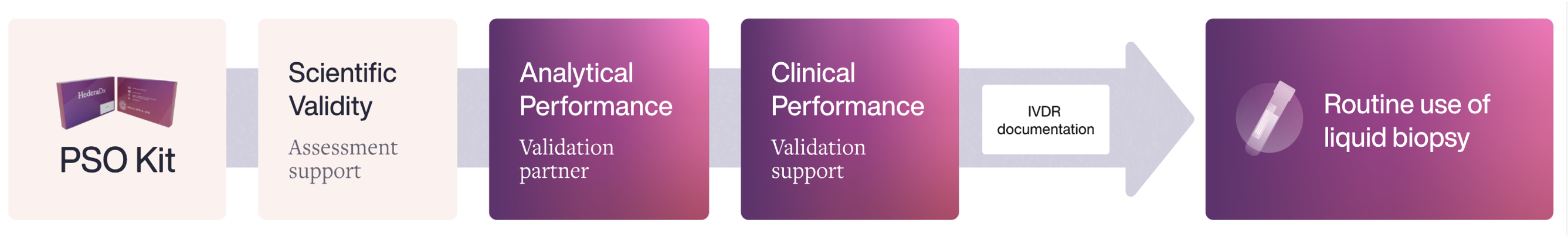
Discover Hedera Comply, a tailored expert IVDR implementation program provided by Hedera Dx to help labs validate Hedera Profiling 2 ctDNA test panel and reach local compliance for routine testing with in-house validation and pre-filled IVDR documentation support.
Specifications
• Panel size: 90 kb
• Instruments supported: Illumina NextSeq and NovaSeq Series
• Multiplexing: up to 6 samples on a NextSeq 500/550 instrument (MID Output flow cell)
• Sample size: min 5mL plasma required (cfDNA BCT by Streck recommended)
• cfDNA input: min 10 ng cfDNA, 30 ng cfDNA recommended
• Library preparation method: hybrid-capture chemistry
• UMI/UDI technologies: allowing error correction and preventing index hopping for enhanced sensitivity and specificity
Hedera Dx’s solution streamlines cfDNA sample-to-report workflows
Hedera Prime radically reduces NGS reporting turnaround time by matching genomic alterations in tumor samples with the appropriate regulatory-approved therapy options.

Powered by Hedera Prime software analysis
Hedera Dx: A validation partner for the Lab
Hedera Comply: tailored expert IVDR implementation program for local compliance, facilitating routine testing with in-house validation and technical documentation support
Comprehensive implementation program to facilitate the integration of compliant liquid biopsy in your lab.
The program provides onsite laboratory hands-on training, including a wet-lab instruction, a run assessment, a software training as well as a validation and technical support.
Supportive technical documentation is also supplied as pre-filled folder to facilitate your local compliance.
Contact – Responsible Manager
Kristýna Slabá
Business Development Manager – Oncology
E-mail: slaba@3genes.com
Mobile: +420720358655
